
| Home |
| Glossary |
| Lobstering |
| Some Facts |
| Handling Gear |
| Wildlife |
| Tools |
| A Day at Sea |
| The Boat |
| Lobsters |
| Facts |
| Molting |
| Eggers |
| Oversized |
| Lobster Sex |
| The Lobster Conservancy |
| The Sea |
| About Tides |
| High Low Tides |
| Misc |
| Credits |
| Site Policy |
| The Author |
Anim. Behav., 1990,39,1199-1206
Moult staggering and serial monogamy in American lobsters, Homarus americanus
DIANE F. COWAN & JELLE ATEMA
Boston University Marine Program, Marine Biological Laboratory, Woods Hole, MA 02543, U.S.A.
Abstract- A mating system of serial monogamy is described for the American lobster, based on observations in large naturalistic aquaria. All except one of the receptive females staggered the timing of their moults at intervals throughout the summer so that each moulted and subsequently mated inside the shelter of a dominant male. In one experiment where there was no clear male dominance, the females moved back and forth from one male shelter to the other before 50% mated with one male and 50% with the other. Cohabitations lasted from 1 to 3 weeks and included periods of both pre- and postmoult shelter sharing. Moult staggering implies behavioural control over the female moult cycle.
The American lobster has served as a useful model for studying the hormonal modulation of motor patterns (Kravitz 1988) and moulting (Chang 1985). While using lobsters for a model of pheromone communication (Atema 1986), we discovered an unusual mating system of serial monogamy and moult staggering. The hormonal regulation of moulting is sensitive to exogenous cues such as temperature, photoperiodicity and food availability (Aiken 1980). In a field population, synchronous moulting was observed in predominantly premoult females after a period of temperature and photoperiod increase (Karnofsky et al. 1989b). Since moulting is coupled to mating (Templeman 1934; Atema et al.1979; Karnofsky et al. 1989b), we hypothesize that access to males may influence the timing of moulting in mature females.
Lobsters live in solitary shelters except when a female moves into a male shelter where she moults and then mates (Atema et al.1979; Karnofsky et al. 1989b). The female, moulting inside the male shelter, benefits from male protection in the vulnerable soft-shelled condition following the moult (Atema & Cobb 1980; Cowan & Atema, unpublished data). The male may benefit through paternity assurance, since the seminal receptacle is cast and any sperm from a previous mating are lost during ecdysis.
Large females who moult infrequently can store sperm for at least 3 years and fertilize multiple batches of eggs (Waddy & Aiken 1986). Fertile eggs are carried for about 11 months before hatching (Templeman 1936)."Therefore, in any breeding season" females can either pair bond, moult and mate, or forego moulting and extrude eggs using sperm from a former mating. There are also two options for males in the breeding season: a male is either dominant and will forego moulting and mate with moulted females, or is subordinate and will moult. By moulting, a mature lobster increases its body weight by about 50% (Aiken 1980), thereby, we suggest, increasing his potential for becoming dominant, since dominance is size dependent (Scrivener 197 1; Atema & Cobb'l 980).
During two mating seasons, we conducted four observational experiments on courtship and mating behaviour in lobsters. By recreating field-fike conditions and observing lobster behaviour in the laboratory where we could see directly inside the lobster's shelters, we gained insight into aspects of social behaviour that are difficult to observe in the field where nocturnal lobsters inhabit deep, dark crevices. Much of lobster reproductive behaviour, including mating, takes place inside shelters. In the field, it has been impossible to observe mating behaviour and only certain components of courtship have been observed (Kamofsky et al. 1989b). However, inferences for the field could be made based on sporadic field observations and on what has been learned in the laboratory.
We describe an unusual mating system exclusive to the American lobster. Although in some other crustaceans the female moults just prior to mating, female lobsters stagger their moults in such a way that males have serial access to receptive females in each breeding season. The physiological implications of moult staggering in lobsters include behavioural control over sexual receptivity and moulting, both of which are endocrine functions. In contrast to many crustaceans, lobsters are long lived and over the course of a lifetime, females undoubtedly cohabit with many males in successive breeding seasons. Similar mating systems have been described as serial monogamy in birds and mammals (Wittenberger 1979; Wittenberger & Tilson 1980).
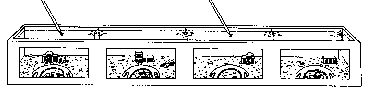
Figure 1. Diagram of the observation aquarium measuring 6 x 1 x .65m, including
seven lobsters, drawn to scale. The arrows indicate sites of seawater
inflow and outflow.
METHODS
A total of seven male (0-6) and 21 female (1-21) lobsters (see Table 1), trapped by Woods Hole fishermen, were observed in 5600-litre aquaria (Fig. 1). To mimic field conditions, the aquaria were stocked with organisms which would normally live near lobster shelters. Hand-moulded concrete shelters, each with two entrances, were pressed against the observation windows. These shelters are preferred by lobsters and will be referred to as primary shelters. Cinderblocks provided additional shelter in the rear of the tank; a coarse gravel substrate, rocks and a variety of marine macroalgae covered the bottom. Various echinoderms, arthropods, molluscs, fish and bryozoans were stocked as live prey. Pieces of dead squid and fish were added several times each week as additional food. Unfiltered sea water flowed through the aquarium at 25 litres/min. Six 60-W incandescent bulbs, sus- pended 30 cm above the aquarium, provided a naturally timed, seasonally adjusted light:dark cycle. Four 15-W bulbs remained on continuously for nocturnal observations.
All lobsters were sexually mature, with a carapace length of between 74 and 81 mm (Hughes & Matthiessen 1962; Atema et al. 1979). The males in each tank were of equal size at the beginning of each experiment. Lobsters were banded behind the dactyl for identification. The claws were unrestrained.
Two male (0 and 1) and five female lobsters (1, 2, 3, 4 and 5) were introduced into a 5600-litre aquarium on 3 June 1983 (experiment A). Male 0 moulted on 7 June 1983; he was removed on 27 June 1983 and replaced by male 2 to maintain the operational sex ratio. Freshly moulted adults avoid conspecifics for about 2 months while the carapace hardens. Therefore, hard-shelled male 2 was introduced to provide a potential mate for the females as well as a competitor for male 1. Female 1 was replaced by female 6 on 27 June 1983 and female 2 by female 7 on 27 July 1983, to hold the operational sex ratio constant. Once a mature female has moulted and mated, she will not moult again for at least a year. Although changing the animals caused some social disruption, it occurs regularly in field populations as animals move into and out of resident populations (Karnofsky et al. 1989a).
Experiments B and C were simultaneous replicates (27 May-31 August 1987). Two male and five female lobsters were introduced into each of two separate 5600-litre aquaria on 27 May 1987 (Table 1). No animals were removed or replaced during experiments B or C. In experiment D (1 September - 17 November 1987), we investigated male moult patterns by leaving the two males (male 5, who did not moult and male 6, who moulted 12 June) from experiment C in the aquarium. Female 17, who did not moult in experiment C also remained in the tank. Four additional females (18, 19, 20 and 21) were added to replace the four postmoult females on 1 September 1987.
| Sex-ID | Premoult CL | Moult date | Postmoult CL |
|---|---|---|---|
| Experiment A | |||
| M-0 | 79 mm | 7 June l983 | 90 mm |
| M-1 | 80 mm | 20 December 1983 | 93 mm |
| M-2 | 80 mm | 6 August 1983 | 92 mm |
| F-1 | 81 mm | 18 June 1983 | 93 mm |
| F-2 | 74 mm | 26 June l983 | 85 mm |
| F-3 | 78 mm | 1 July 1983 | 89 mm |
| F-4 | 81 mm | 18 July 1983 | 91 mm |
| F-5 | 78 mm | 4 August 1983 | 88 mm |
| F-6 | 79 mm | 15 July 1983 | 90 mm |
| F-7 | 79 mm | 5 September 1983 | 89 mm |
| Experiment B | |||
| M-3 | 77 mm | Not in 1987 | |
| M-4 | 77 mm | 27 July 1987 | 88 mm |
| F-8 | 77 mm | 26 June l987 | 85 mm |
| F-9 | 79 mm | 26 July 1987 | 90 mm |
| F-10 | 75 mm | 12 August 1987 | 85 mm |
| F-11 | 81 mm | 27 September 1987 | 92 mm |
| F-12 | 78 mm | Not in 1987 | |
| Experiment C | |||
| M-5 | 75mm | Not in 1987 | |
| M-6 | 75mm | 12 June l987 | 85mm |
| F-13 | 78mm | 12 June ]987 | 87mm |
| F-14 | 77mm | 9 July 1987 | 86mm |
| F-15 | 77mm | 13 July 1987 | 88mm |
| F-16 | 77mm | 25 July 1987 | 87mm |
| F-17 | 74mm | 1 October 1987 | 83mm |
| Experiment D | |||
| M-5 | As above | ||
| M-6 | As above | ||
| F-17 | As above | ||
| F-18 | 78mm | 22 September 1987 | 88mm |
| F-19 | 78mm | 27 September 1987 | 88mm |
| F-20 | 76mm | 24 October 1987 | 85mm |
| F-21 | 77mm | Not in 1987 |
ID: identification number; M: male, F: female; CL: carapace length.
Data were collected every day by the following methods: (1) focal observations concentrating on each individual for 10 min twice per day, (2) census maps recording the location of each lobster at least four times and up to 24 times (X + SE= 12 +- 0.3) per day at 1-h intervals, and, in experiment A only, (3) nightly long-term observations focusing on mated pairs. Long-term observations lasted up to 18 consecutive hours and totalled 183 h in 1983 and 205 h in 1987.
Each stage of cohabitation was defined as follows (after Atema et al. 1979): phase 0, no shelter sharing; phase 1, intermittent premoult shelter sharing; phase 2, permanent premoult shelter sharing; phase 3, female moult; phase 4, permanent postmoult shelter sharing; and phase 5, intermittent postmoult shelter sharing. Intermittent refers to part-time, and permanent to full-time female residency in a male shelter during all observation times.
The amount of shelter checking and pleopod fanning (in experiment A), and the number of aggressive acts (in experiments A-D) were recorded. Shelter checking included any incident where a lobster approached within a few cm of another lobster's shelter entrance. To standardize data, the number of shelter checks was divided by the total number of observation hours per day for each individual.
Pleopod fanning creates a strong current pulling water toward and propelling water beneath and behind the lobster. The daily frequency of male pleopod fanning was categorized as follows: 0, no fanning; 1, fanning once and for less than 1 min; 2, fanning at least twice but not in more than half of the censuses; 3, fanning in more than half, but not all of the censuses; 4, fanning in all censuses and all other observations. A chi-squared contingency test was performed to compare pleopod fanning frequencies in cohabitation phases 2, 3 and 4 versus 0, 1 and 5.
In aggressive acts the animal who retreated, fled, backed away or tail flipped was considered the loser. Males were then ranked according to the number of wins and losses. The lobster who won consistently was regarded as dominant. In experiments B and C, we saw few interactions. Therefore, we assessed dominance on the basis of shelter use where a male residing in a primary shelter(s) was assumed to be dominant over a male residing in a cinderblock or in the open. In experiment D, we saw too few encounters to assess dominance and both males lived in primary shelters. Chi-squared goodness-of-fit analyses were performed to determine whether females showed a preference for mating with the dominant male.
RESULTS
Colhabitations lasted from 7 to 27 days and included a period of pre- and postmoult shelter sharing. The receptive females in experiments A, B and C, precisely staggered the timing of their moulting throughout the summer to mate with the dominant male (Fig. 2a-c). The number of days each pair spent in cohabitation varied.
In experiment A, male 1 won 42 (91.3%) of the 46 aggressive encounters observed between males 1 and 2 during 1983 and was therefore considered dominant. Each female, except female 6, initiated a pair bond, cohabited, moulted and subsequently mated with the dominant male (Fig. 2a). Female dominance was not related to moult order. Female 6 cohabited with the subordinate male 2. This happened while male 1 was cohabiting with female 3 and then female 4. Thus, females showed a preference for mating with the dominant male (X^2=3.57,df=l, P<0.05).
Cohabitation overlap occurred once in experiment A, when female 4 began intermittent premoult cohabitation with the dominant male while the previous female (3) was temporarily outside of the dominant male shelter on her final day of intermittent postmoult cohabitation. Two females never occupied the dominant male shelter at the same time. The subordinate male moulted on 6 August 1983; the dominant male moulted on 20 December 1983.
In experiment B, the dominant male (3), cohabited and mated with females 8-11, in sequence (Fig. 2b). Male 3 won three out of three aggressive acts observed between males 3 and 4. In all three interactions male 3 succeeded in evicting male 4 from his shelter. Furthermore, male 4 was less faithful to one shelter (perhaps due to evictions) and was often found outside with no shelter. Thus, male 3 was considered dominant. Again, the females showed a mating preference for the dominant male (X^2 = 3.40, df= 1, P < 0.05). Female 12 did not pair bond or moult; instead, she extruded eggs and attached them to her pleopods. The subordinate male moulted on 27 July 1987 and did not cohabit with any female. After moulting, male 4 was considered subordinate because, as is usual for moulted lobsters, he avoided all other lobsters. The dominant male (3) did not moult during 1987.
In experiment C the dominant male (5) cohabited and mated with females 13-16, in sequence (Fig. 2c). Although no aggressive interactions were observed, male 6 was considered subordinate prior to moulting because he lived in a secondary shelter or in the open, while male 5 lived in a primary shelter. Again, the females preferred the dominant male (X^2 = 3.40, df= 1, P < 0.05). Female 17, like female 12 in experiment B, did not pair bond or moult, but, rather, extruded eggs. Male 6 moulted on 12 June 1987 and was then considered subordinate because he avoided all other lobsters.
As a result of moulting during experiment C, male 6 became 10% larger in carapace length (and about 50% heavier; see Aiken 1980) than male 5 and we expected the mating preference to shift after his exoskeleton calcified. Female mating preference did not shift entirely: 2 months after male 6 moulted, two females in experiment D mated with male 6 while the other two chose male 5 (Fig. 2d) showing no consistent mating preference, in contrast to experiments A-C.
In experiment D, all four of the females who moulted and mated in autumn switched between males by moving back and forth from one male shelter to the other during premoult cohabitation. Each mated with only one of the two males and remained in his shelter for the postmoult cohabitation period. Surprisingly, the pregnant female, female 17 from replicate C, mated with male 6 after casting viable eggs (i.e. live, well-developed embryos) along with her moult shell. Female 17 had not moulted in the tank prior to her cohabitation with male 6. We presume that she fertilized the eggs using sperm stored from a previous mating. Female 21, like female 12 in experiment B and 17 in experiment C, extruded eggs thus foregoing moulting. Male 5 did not moult during 1987.
During experiment A, male pleopod fanning frequency increased with progressive phases of cohabitation, peaked on the day of the female's moult, and declined on the days following mating (Fig. 3). The number of times the females checked the dominant male's shelter correlated with the frequency of male pleopod fanning. A significant increase in male pleopod fanning frequency occurred during permanent shelter sharing versus intermittent and no shelter sharing (X^2=7.97, df=],P<0.001). When not cohabiting, both males and females fanned irregularly.
DISCUSSION
In the laboratory, we observed moult staggering in mature, sexually receptive females that resulted in a mating system of serial monogamy. In contrast, a field population of mostly immature females moulted synchronously in June (Karnofsky et al. 1989a). We hypothesize that the timing of moulting in mature females is related to the availability of dominant males, a condition that would not influence the timing of moulting in immature animals. Moult staggering may be a function of population density, sex ratio, female dominance and/or the availability of mates.
The sequential timing of female moulting and cohabitation with the dominant male lobster (Fig. 2) is best described as a mating system of serial monogamy. While the mechanisms that regulate the timing of mature female moulting are not understood at present, we can speculate on two possible functions of moult staggering: (1) it increases the mating success of the dominant male and (2) it allows females to mate with the dominant male who is presumably more capable of protecting her from predation and cannibalism in the vulnerable, soft-shelled state immediately following her moult.
Skewing the sex ratio toward females in our naturalistic aquaria may have emphasized the sequential timing of female moults, since each female delayed moulting until pair bonding with the dominant male. Fishing statistics show a 1:1 sex ratio (Skud & Perkins 1969) in the field. However, it is likely that dominance in males results in a functional bias of the operational sex ratio in favour of females, thereby leading to serial monogamy and moult staggering. Karnofsky et al. (1989b) observed the consecutive cohabitations of two females with one male in a natural field population. This observation is consistent with our laboratory findings.
The factors determining the order of female moulting (i.e. who moults first and who waits until last) are unknown. Moult order was not related to female dominance order. In the same aquaria, skewing the sex ratio toward males (four males and two females) resulted in high aggression and an inability for any male to establish dominance. Consequently, females delayed ecdysis indefinitely and moved into and out of up to three male shelters per day, perhaps unable to choose a suitable mate (Cowan & Ellis, unpublished data). Both pre- and postmoult cohabitation periods were much longer, perhaps due to increased intrasexual male competition. In contrast, shorter premoult cohabitations were observed with a 1:1 sex ratio (Atema et al. 1979), perhaps due to decreased female competition.
A competitive male strategy may be 'out-moulting' the competition, since the moulted male becomes about 50% larger in body weight and may therefore begin to win previously futile aggressive encounters. However, for about 2 months after moulting, postmoult males avoid encounters with conspecifics. In experiments A, B and C, where the males were originally of equal size, the subordinate male moulted first. The females, therefore, had no choice but to mate with the dominant male or to forego moulting and extrude eggs. It was only after the moulted male's carapace hardened in experiment D, that females began to move back and forth from one male shelter to the other before finally pair bonding, moulting and mating with one of the males (Fig. 2d). It may have been difficult for females to assess dominance in these males. Karnofsky & Price (1989) observed similar behaviour in what they termed 'fickle females'. Interestingly, the egg bearing (pregnant) female (17), in experiment D (Fig. 2d), moulted, casting off viable eggs and mated with the previously subordinate male.
Moult staggering in females may be influenced by chemical communication. The more frequently the male 'pleopod fans', the more frequently the females 'shelter check' (Fig. 3). Fanning disperses the scent of the shelter water, which bears the odour of the pair. If the odour of the mating shelter attracts mates, as we suspect it does based on the relationship between fanning and checking, then we propose the existence of primer pheromones that may facilitate female choice and regulate female moulting.
ACKNOWLEDGMENTS
We thank Rainer Voigt for computer programming and Michelle Scott for critical review of an earlier version of this manuscript. We also thank Carl Merrill, Leslie Sammon, Caroline Radding and Frank Corrotto for assisting with census.and focal observations. Partial financial support for this study was provided by the Whitehall Foundation and NSF (BNS8413661) to J.A.
REFERENCES
Aiken, D. E. 1980. Molting and growth. In: The Biology and Management of Lobsters. Vol. I (Ed. by J. S. Cobb & B. F. Phillips), pp. 91-163. New York: Academic Press.
Atema, J. 1986. Review of sexual selection and chemical communication in the lobster, Homarus americanus. Can. J. Fish. Aq. Sci., 43,2283-2390.
Atema, J. & Cobb, J. S. 1980. Social behavior. In: The Biology and Management of Lobsters. Vol. I (Ed. by J. S. Cobb & B. F. Philips), pp. 409-450. New York: Academic Press.
Atema, J., Jacobson, S., Karnofsky, E., Oleszko-Szuts, S. & Stein, L. 1979. Pair formation in the lobster, Homarus americanus. Mar. Behav. Physiol., 6,277-296.
Chang, E. S. 1985. Hormonal control of molting in decapod Crustacea. Am. Zool., 25, 179-185.
Hughes, J. T. & Matthiessen, G. C. 1962. Observations on the biology of the American lobster, Homarus americanus. Limnol. Oceanogr., 7,414-421.
Karnofsky, E. B., Atema, J. & Elgin, R. H. 1989a. Natural dynamics of population structure and habitat use of the lobster, Homaru.v amt,rirantiv, in a shallow cove. Biol. Bull., 176,247-256.
Karnofsky, E. B., Atema, J. & Elgin, R. H. 1989b. Field observations of social behavior, shelter use and foraging in the lobster, Homarus americannus. Biol. Bull., 176,239-246.
Karnofsky, E. B. & Price, H. J. 1989. Dominance, territoriality and mating in the lobster, Homarus americanus: a mesocosom study. Mar. Behav. Physiol., 15,101-121.
Kravitz, E. 1988. Hormonal control of behavior: amines and the biasing of behavioral output in lobsters. Science, 241, 1775-1781.
Scrivener, J. C. E. 1971. Agonistic behavior in the American lobster, Homarus americanus. Fish. Res. Bd Can. Tech. Rep., 235,1-115.
Skud, B. E. & Perkins, H. C. 1969. Size composition, sex ratio, and size at maturity. U.S. Fish. Wildl. Serv., Spec. Sci. Rep., 598, 1 -1 0.
Templeman, W. 1934. Mating in the American lobster. Contr. Can. Biol. Fish., 8,422-432.
Templeman, W. 1936. Local differences in the life history of the lobster (Homarus americanus) on the coast of the maritime provinces of Canada. J. Biol. Bd Can., 2, 41-88.
Waddy, S. & Aiken, D. E. 1986. Multiple fertilizations and consecutive spawning in large American lobsters, Homarus americanus. Can. J. Fish. Aq. Sci., 43, 2291-2294.
Wittenberger, J. F. 1979. The evolution of mating systems in birds and mammals. In: Handbook of Behavioral Neuroscience.- Social Behavior and Communication (Ed. by P. Marler & J. Vandenbergh), pp. 271-349. New York: Plenum.
Wittenberger, J. F. & Tilson, R. L. 1980. The evolution of monogamy: hypotheses and evidence, A. Rev. Ecol. Syst., 11, 197-232.

